Current Projects
- Genetics and Genomic of Pulmonary Fibrosis
- Genetics of Innate Immunity and Host Defense
- Epigenetic Regulation in Asthma
- Children's Environmental Health Center
Genetics and Genomic of Pulmonary Fibrosis
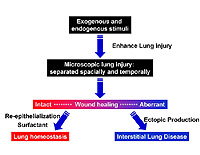
Pulmonary fibrosis is a general term used to describe the group of fibrosing interstitial lung diseases that are characterized by progressive scarring of the alveolar interstitium, often leading to hypoxemic respiratory insufficiency. Pulmonary fibrosis can result from environmental exposures, or occur as an isolated, sporadic disease without extrapulmonary involvement (idiopathic interstitial pneumonia or IIP). IIPs are composed of several subtypes of pulmonary diseases that differ in clinical, radiographic, and histopathologic presentation; while some have a constellation of specific features that allows a clear diagnosis to be made, all too frequently the type of IIP can't be characterized and many of the subtypes of IIP have overlapping clinical and laboratory features indicating that the current definitions remain too broad. There is also emerging evidence that the development of all forms of interstitial pulmonary fibrosis is, at least in part, determined by genetic factors.
Our pulmonary fibrosis research seeks to uncover the genetic and environmental determinants of IIP. Our ongoing effort to establish the etiology of this complex human disease involves high-throughput resequencing and genotyping, whole genome sequencing, gene expression and miRNA profiling, and defining the IIP methylome. Our ultimate goal is to identify candidate susceptibility genes, explore relationships between specific environmental exposures and candidate susceptibility genes, and define the unique molecular phenotypes in pulmonary fibrosis.

We are currently performing a whole genome association study (GWAS) in 5000 cases of fibrosing interstitial lung disease (fILD) and a linkage study in over 200 families with familial interstitial pneumonia (FIP) to identify novel genes that regulate fibroproliferative response in the lung of individuals with IIP. In addition, we are combining genetic and genomic findings in patients with IIP to develop and validate phenotypically-anchored molecular signatures that will serve to refine the diagnostic criteria for this group of complex diseases. To this end, we are profiling 500 cases of IIP on gene expression arrays and miRNA arrays and combining genomic data with GWAS data on these subjects to establish genomic signatures for specific subtypes of IIP, to establish expression quantitative trait loci (eQTLs), and identify miRNAs that may regulate fibroproliferative response in the lung.
Candidate genes that are identified using genetics and genomic approaches in humans are examined for their functional role in pulmonary fibrosis using mouse lines with targeted mutations and transgenes, and established mouse models (bleomycin, environmental tobacco smoke, asbestos etc).
Genetics of Innate Immunity and Host Defense
Innate immunity is conserved over a wide variety of species from flies to mammals. Innate immunity uses germline-encoded receptors to aid in anti-microbial host defense, with Toll-like receptors (TLRs) being the most extensively studied family. These receptors recognize certain patterns, rather than particular structures, thereby allowing a limited number of Pattern Recognition Receptors (PRRs) to recognize a wide variety of microbes. In the case of LPS, the PRR is directed toward the highly conserved portion of LPS known as lipid A. As such, lipid A is considered the Pathogen Associated Molecular Pattern (PAMP) for the PRR TLR4.
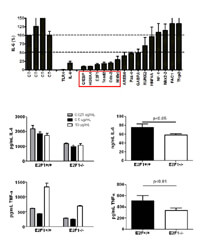
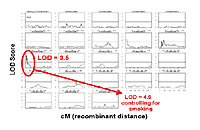
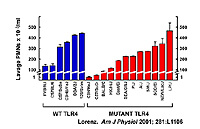
We have previously shown that polymorphisms in TLR4, the receptor for LPS, are associated with hyporesponsiveness to inhaled LPS in mice and humans. We have also shown that these same polymorphisms predispose humans to Gram negative sepsis and protect them from atherosclerosis. However, our previous findings also demonstrate that sequence variants of TLR4 account for only a portion of the LPS phenotype in either mice or humans and that other genes are also involved in regulating the response to LPS.
One of the goals of the innate immunity project is to identify novel genes involved in innate immunity and consequently determine if polymorphisms in some of these genes regulate the innate immune response to Gram negative sepsis and other microbial infections in humans. Novel genes are identified using genetic mapping (whole genome association and quantitative trait loci mapping) and expression studies in mice and cultured macrophages stimulated with bacterial, viral, and fungal PAMPs. Candidate innate immune genes identified using these approaches are tested functionally by RNA interference in macrophages and in mouse lines with targeted mutations. Polymorphisms in human orthologs of candidate genes are then tested in patient cohorts.
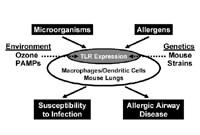
Another major goal of the innate immunity project is to understand how and why environmental exposures such as air pollution alter lung host defense. To do this, we are testing the hypothesis that the expression of toll-like receptors (TLRs) in the lung are influenced by environmental (ozone and/or PAMPs) and genetic factors, and the dynamic expression of TLRs has profound effects on lung host defense and consequently the development of lung infections and allergic airway disease. We are conducting a series of in vitro experiments to identify TLRs whose expression is altered as a result of ozone and/or PAMP exposure in bone marrow derived macrophages and dendritic cells from five strains of mice with previously established sensitivity to ozone and LPS. We will then test whether exposures that led to altered TLR expression on specific cell types in vitro also alter expression of the same TLRs in relevant cell types in the lungs of mice. Finally, we will examine the impact of altered TLR expression in mouse lungs on host defense/innate immune response to relevant lung pathogens and development of allergic airway disease.
Epigenetic Regulation in Asthma
Asthma is the most common chronic childhood disease, affecting 9 million children in the United States. This disease is increasing in prevalence, incidence, and severity, particularly in developed countries. Asthma is a strongly familial condition, with estimates of heritability ranging from 36 - 79 percent. Although genetic factors unquestionably play a role in the development of asthma and atopy, the environmental factors to which children are exposed in utero and postnatally are also of considerable importance. The overall hypothesis of our project is that epigenetic mechanisms play a fundamental role in the etiology of asthma by modulating transcriptional activity of critical genes that affect immune maturation through alterations in DNA methylation and chromatin modification status, and this then leads to the development of asthma.
Methylation of CpG islands within regulatory regions of DNA is an important epigenetic mechanism controlling transcriptional activity, with the level of CpG methylation within specific genetic regions being heritable. It has been demonstrated that the concentration of methyl donors in the diet of pregnant agouti mice has transgenerational effects on coat color of the progeny due to altered expression of the agouti gene, a result of methylation of the agouti promoter. Emerging research indicates that epigenetic mechanisms affect the expression of transcription factors that control the lineage of Th1, Th2, and Treg cells.
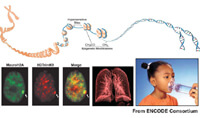
One of the approaches we are taking in our laboratory is to study the role in utero diet and environmental exposures play in the development of allergic airway disease in mice, to study how DNA methylation and chromatin modifications are altered by these exposures in specific immune cell types and, and how these changes consequently lead to the development of asthma and atopy. Our recently published findings indicated that in mice, a maternal diet supplemented with methyl-donors enhanced the severity of allergic airway disease that was inherited trans-generationally. Using methylation-specific digital karyotyping (MSDK), we discovered 82 gene-associated loci that were differentially methylated after in utero supplementation with a methyl-rich diet rich. This study provided the first in vivo evidence that in utero diet can impact CpG methylation and trans-generational inheritance of allergic airway disease. We are currently pursuing other in utero diets and exposures (folate concentrations, environmental tobacco smoke etc) and alternative, more comprehensive genome-wide approaches to study DNA methylation and histone modifications, specifically, CHARM microarrays and CHIP-seq.
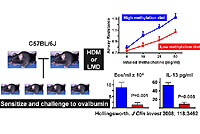
In addition to the murine studies, we are examining DNA methylation patterns in children with asthma. We are conducting a number of studies to elucidate the role of DNA methylation in cohorts of children with asthma. In one study (Figure 10A), we will measure global methylation patterns in a panel of human sibling pairs discordant for asthma (MRC National Collection of Asthma Families (MRCA), consisting of 206 British families that were ascertained through an affected proband with at least Stage 3 asthma; collaboration with Drs. Bill Cookson and Miriam Moffat) (Aim 1), and in airway epithelia and peripheral blood mononuclear cells (PBMCs) obtained from patients with asthma and controls (Aim 2). We will combine these global methylation marks with our knowledge of global methylation patterns in mouse lungs with allergic airway disease(10), and then confirm these methylation patterns with direct sequencing (Aim 3). We will then validate and further characterize these findings in two independent study populations. Using the original discordant sibling pair sample and their parents, we will test whether these targets are imprinted (Aim 4). Using a large, independent sample of asthmatic cases and controls for which extensive environmental and genetic data are available, we will test whether these targets replicate and whether they are influenced by either environmental or genetic factors (865 asthmatics and 865 control primary school children age 6 - 13 years in rural areas of Bavaria, Baden-Württemberg, Tyrol, and Switzerland; collaboration with Dr. Erika von Mutius) (Aim 5).
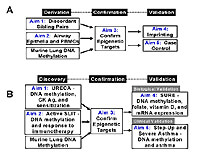
In another study (Figure 10B), we plan to determine the extent to which epigenetic mechanisms contribute to the etiology of asthma in children living in the inner city (Inner City Asthma Consortium). In this project, we will identify CpG islands that are differentially methylated in response to gestational cockroach antigen or are associated with development of sensitization or episodic wheezing in inner city children (Aim 1), identify CpG islands that are differentially methylated in response to active cockroach sublingual immunotherapy (SLIT) among 100 mild to moderate asthmatics, confirm methylation changes associated with asthma or allergic airway disease (prior murine study (10)) detected by sequencing DNA in selected study subjects (Aim 3), determine whether differentially methylated genes found to be associated with allergic disease, cockroach exposure, or cockroach immunotherapy in are affected by cord blood concentrations of folate or vitamin D (Aim4), and determine whether differentially methylated genes found to be associated with allergic disease, cockroach exposure, or cockroach immunotherapy are associated with the presence and severity of asthma (Aim 5). Identifying unique epigenetic marks and linking them to other etiologic factors, such as environmental exposures and genetic variants, will transform our understanding of the etiology and pathogenesis of asthma and lead to more effective approaches to disease prevention and treatment.
Children's Environmental Health Center (CEHC)
The theme of the Denver Children's Environmental Health Center (CEHC) is to investigate the etiology and pathogenesis of airway disease in children. We chose this theme for our program for the following reasons:
- Asthma and other forms of airway disease/illness are the most common chronic illnesses in children.
- The environment results in unique combinations of exposures to children that affect lung immunity and consequently alters the risk of developing airway disease.
- Environmental models of airway disease provide an ideal opportunity to investigate basic mechanisms involved in the development of childhood airway disease such as the basic immunology and persistence of airway disease.
- This theme builds on existing scientific expertise and relationships with community stakeholders and members to ensure a highly interactive program.
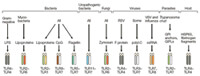
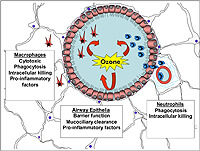
Since ozone and endotoxin are common environmental exposures, and both of these agents have been demonstrated to have immune modulating affects (Figures 1 and 2), we have decided to use these very relevant environmental exposures to further focus the projects in our proposed program. The end result is a highly integrated and focused Center that has the potential to make a number of novel, related observations. In aggregate, the coupled scientific findings from the proposed program will substantially enhance our understanding of airway disease in children that can be immediately translated to communities in Colorado.
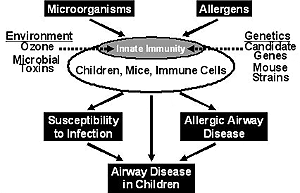
The overarching hypothesis that unifies this research program is that lung host defense and lung immunity are dynamic biologically, are affected by ozone and endotoxin, and that this dynamic biology can alter susceptibility to agents known to cause airway disease in children (Figure 3).
We believe that understanding the basic and translational aspects of this hypothesis will provide the scientific rationale to develop primary, secondary and tertiary prevention programs that reduce the morbidity and mortality of childhood airway disease/illneses.
Our Project-Specific Hypotheses
 Project 1 : Higher levels of endotoxin exposure cause persistent, problematic asthma and that key environmental (ozone and allergens) and genetic modifiers (endotoxin receptor polymorphisms) contribute to endotoxin susceptibility and pathological asthmatic responses.
Project 1 : Higher levels of endotoxin exposure cause persistent, problematic asthma and that key environmental (ozone and allergens) and genetic modifiers (endotoxin receptor polymorphisms) contribute to endotoxin susceptibility and pathological asthmatic responses.
Project 2: Ozone exposure in the early postnatal phase alters lung development and modifies the host immune response to early life viral infection and allergen exposure, thereby contributing to the development of reactive airway disease.
Project 3: Expression of toll-like receptors (TLRs) in the lung are influenced by environmental (ozone and/or PAMPs) and genetic factors, and the dynamic expression of TLRs has profound effects on lung host defense and consequently the development of lung infections and allergic airway disease. Community Outreach and Translation Core: This core serves as a forum for dialoguing and exchanging information among investigators, practitioners, and community stakeholders regarding the activities of the Children’s Health and Environment Center. The overarching hypothesis of this core is that a community-based outreach program can effectively educate children to modify environmental exposures that cause respiratory illnesses and associated morbidity.
