Epigenetics
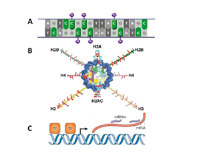 Despite having identical genomic sequences, different cell types exhibit substantially different profiles of gene expression. Cell-specific gene-expression patterns are specified and maintained through epigenetic regulation. Epigenetics is the stable and heritable information that is distinct from DNA sequences and fostered by specialized mechanisms, including DNA methylation, small interfering RNAs, and histone post-translational modifications. In addition to being heritable, epigenetics is influenced by aging, environment, and in utero exposures. Epigenetic mechanisms have long been associated with development of cancer1 but the role of epigenetic regulation in non-malignant diseases is just emerging2,3.
Despite having identical genomic sequences, different cell types exhibit substantially different profiles of gene expression. Cell-specific gene-expression patterns are specified and maintained through epigenetic regulation. Epigenetics is the stable and heritable information that is distinct from DNA sequences and fostered by specialized mechanisms, including DNA methylation, small interfering RNAs, and histone post-translational modifications. In addition to being heritable, epigenetics is influenced by aging, environment, and in utero exposures. Epigenetic mechanisms have long been associated with development of cancer1 but the role of epigenetic regulation in non-malignant diseases is just emerging2,3.
Role of Epigenetics in Complex Lung Diseases
In the Center for Genes, Environment & Health, we are studying the role that epigenetic regulation may play in the etiology of several lung diseases, including asthma, COPD and pulmonary fibrosis.
Asthma
Asthma is the most common chronic childhood disease, affecting 9 million children in the United States. This disease is increasing in prevalence, incidence, and severity, particularly in developed countries.
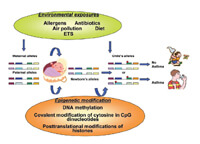 Several lines of evidence support a role for epigenetics in asthma.
Several lines of evidence support a role for epigenetics in asthma.First, asthma, like epigenetic mechanisms, is heritable. While a classic Mendelian pattern of inheritance is not observed in patients with asthma, asthma is heritable, has been linked to various genomic loci, and is associated with polymorphisms in more than 100 genes5. Many of these associations, however, have proven not to be replicable, and together these findings account for only a small proportion of disease heritability. At present, genetics falls far short in explaining the etiology of asthma.
Second, asthma, like epigenetic mechanisms, is influenced by the environment. While allergens are classically associated with asthma, many other exposures, including air pollution, tobacco smoke, microbial toxins, viral infections, and dietary factors are associated with the development and progression of this disease.
Third, asthma, like epigenetic mechanisms, is affected by in utero exposures and aging. Asthma incidence in children has been associated with maternal dietary folate in recent epidemiological studies6,7. Moreover, recent work in mice suggests that in utero diet can have an impact on CpG methylation and trans-generational inheritance of allergic airway disease8.
Fourth, asthma is an immune-mediated disease with skewing toward a Th2 phenotype. The transcription factors involved in the development of mature T cells (Th1, Th2, and Tregs) have been shown to be influenced by epigenetic mechanisms. Finally, the higher (and growing) prevalence and incidence of asthma in developed countries, such as the U.S. and Western Europe, reduces the likelihood that changes in the sequence of DNA account for the full heritability of asthma and, instead, suggest that other mechanisms involved in inheritance, such as epigenetics, contribute to the etiology of this disease.
Role of DNA Methylation in Human Cohorts
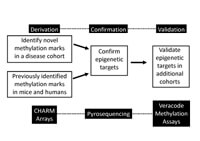
Our general approach to the study of the role of DNA methylation in human cohorts is outlined in. We use genome-wide approaches to the study of DNA methylation, such as CHARM arrays, to identify novel CpG motifs that are hypo- or hyper-methylated in individuals with the disease compared to controls. We combine our novel findings with our previous finding in mice or humans, we confirm differentially methylated regions using pyrosequencing, and finally validate our findings using focused DNA methylation assays (Illumina Veracode assays) in independent cohorts.
Studies in Mice to Understand Molecular Mechanisms
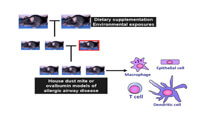 In addition to our work in human populations, we are conducting studies in mice to gain a better understanding of molecular mechanisms that underlie the role epigenetic marks play in the development of Th2 immunity and allergic airway disease. More specifically, we are studying the role of epigenetic marks in the maturation of specific immune cell populations that have been shown to play a role in human asthma. In these studies, pregnant mice are exposed to specific environmental exposures such as tobacco smoke or fed a diet rich in methyl group donors such as folate. The progeny are then phenotyped using mouse models of allergic airway inflammation to determine the effect of in utero dietary modifications and environmental exposures on epigenetic marks (DNA methylation and histone modifications) in specific immune cells (macrophages, dendritic cells, T-cells etc).
In addition to our work in human populations, we are conducting studies in mice to gain a better understanding of molecular mechanisms that underlie the role epigenetic marks play in the development of Th2 immunity and allergic airway disease. More specifically, we are studying the role of epigenetic marks in the maturation of specific immune cell populations that have been shown to play a role in human asthma. In these studies, pregnant mice are exposed to specific environmental exposures such as tobacco smoke or fed a diet rich in methyl group donors such as folate. The progeny are then phenotyped using mouse models of allergic airway inflammation to determine the effect of in utero dietary modifications and environmental exposures on epigenetic marks (DNA methylation and histone modifications) in specific immune cells (macrophages, dendritic cells, T-cells etc).
References
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002;3:415-428.
- Miller RL, Ho SM. Environmental epigenetics and asthma: Current concepts and call for studies. American journal of respiratory and critical care medicine 2008;177:567-573.
- Shaheen SO, Adcock IM. The developmental origins of asthma: Does epigenetics hold the key? American journal of respiratory and critical care medicine 2009;180:690-691.
- Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and micrornas. Nat Rev Genet 2007;8:93-103.
- Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol 2008;8:169-182.
- Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child 2009;94:180-184.
- Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: A prospective birth cohort study. Am J Epidemiol 2009;170:1486-1493.
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific cpg island shores. Nat Genet 2009;41:178-186.
