Clinical Data
Questionnaire: Each participant completes a private interviewer-administered questionnaire to obtain demographics, medical history, clinical symptoms and occupational and exposure history.

Clinical data: Each participant is asked to provide consent to review medical evaluation results and tests.
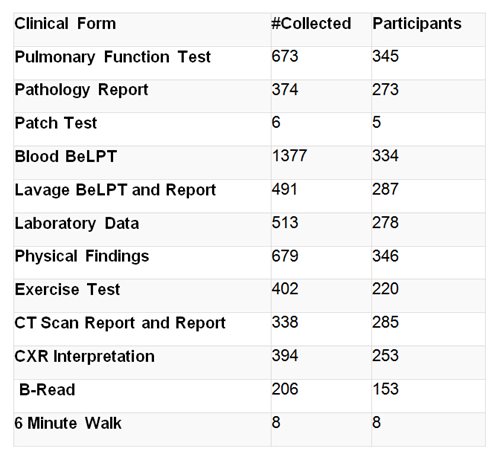
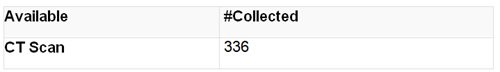
Radiological Data: Each clinical center has obtained copies of CT scan images from their respective radiology department's CT scanners in a format that has de-identified participant-specific data. The DCC has copies of these CT scans available to researchers.
Specimens
The following table lists the current biological specimen inventory available to researchers.
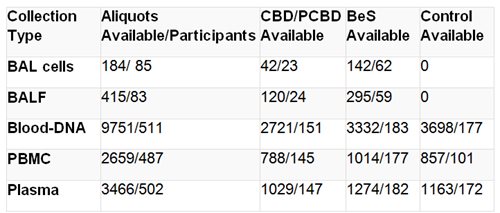
Note: Transbronchial biopsy tissue is also available from 216 participants (3 slides each)
Storage Specifications
Plasma
Plasma samples are split into cryovials – 0.5 ml per vial. They are stored at -80°C in 2 inch fiberboard boxes.
Isolated DNA
DNA has been isolated from the blood and separated into aliquots at a volume of 0.1ml. Please note that the concentration of DNA (µg/ml) per aliquot is variable and requests will be made per volume, not concentration. DNA vials are labeled with printed labels and stored at -80 °C.
Cryopreserved Cells
Cells are split into 5 million cells per aliquot. They are stored in cryogenic safe plastic boxes in liquid nitrogen vapor tanks.
BAL Cryopreserved Cells
Cells are split into 10 million cells per aliquot. They are stored in cryogenic safe plastic boxes in liquid nitrogen vapor tanks.
BAL Fluid
BALF samples are split into cryovials – 3.5 ml per vial. They are stored at -80 °C in the 2 inch fiberboard boxes. Specific processing procedures and storage conditions can be found in the Beryllium BioBank Protocol.
Data & Specimen Requests
Investigators wishing to use BBB data and/or specimens to investigate hypotheses which are directly associated to patients with chronic beryllium disease should submit a written proposal.
All proposals for acquisition of specimens and clinical data will be reviewed based on the statistical plans and power analysis as well as evaluating it for merit and feasibility.
For requests, please contact:
Lisa A. Maier, MD, MSPH, FCCP
Beryllium BioBank
National Jewish Health
1400 Jackson St., G204
Denver, CO 80206
MaierL@NJHealth.org or BarkesB@NJHealth.org