T cell Research
Details
Structure and function of T cell receptors and their role in human diseases
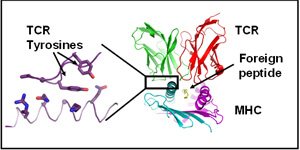 T cells use their aß T cell receptors (TCRs) to detect foreign material in the body. TCRs usually react with peptides derived from the foreign material bound to proteins of the host, the so-called major histocompatibility complex proteins (MHC). The fact that TCRs are so biased towards reaction with MHC has been somewhat of a mystery. Our laboratory has recently found that particular amino acids of TCRs are crucial for their ability to react with MHC. For example, the adjacent figure shows the structure of a TCR (in red and green) reacting with a foreign peptide (in yellow) bound to MHC (in cyan and lavender). A blow up of one of the areas of contact shows that two TCR tyrosines are intimately involved in binding the MHC. This interaction has consistently been seen by others and ourselves in many TCR + peptide + MHC structures. Our research has shown that these two amino acids, when present in TCRs, are critical and moreover, that they have been evolutionarily conserved in TCR sequences since the time that TCRs evolved, about 450 million years ago.
T cells use their aß T cell receptors (TCRs) to detect foreign material in the body. TCRs usually react with peptides derived from the foreign material bound to proteins of the host, the so-called major histocompatibility complex proteins (MHC). The fact that TCRs are so biased towards reaction with MHC has been somewhat of a mystery. Our laboratory has recently found that particular amino acids of TCRs are crucial for their ability to react with MHC. For example, the adjacent figure shows the structure of a TCR (in red and green) reacting with a foreign peptide (in yellow) bound to MHC (in cyan and lavender). A blow up of one of the areas of contact shows that two TCR tyrosines are intimately involved in binding the MHC. This interaction has consistently been seen by others and ourselves in many TCR + peptide + MHC structures. Our research has shown that these two amino acids, when present in TCRs, are critical and moreover, that they have been evolutionarily conserved in TCR sequences since the time that TCRs evolved, about 450 million years ago.
We are using our knowledge of TCR and MHC structures to study several human diseases that are caused by T cell recognition of foreign and self antigens. We are particularly interested in T cell reaction with metal ions such as beryllium and nickel. Beryllium recognition is important because this event leads to a severe, sometimes fatal, human lung disease, berylliosis. This allergic disease is caused by T cell reaction with MHC and beryllium in the lungs of some workers who machine this metal. In collaboration with Dr. Andrew Fontenot and his colleagues at the University of Colorado, Denver, we are trying to understand how TCRs react with the small Be++ ion. Nickel reaction is of interest because it is the most common allergy present in the human population.
The TCRs on T cells sometimes react with peptides of their own host. When this happens the T cells will attack host tissues, thus causing autoimmune diseases such as type 1 diabetes and rheumatoid arthritis (RA). Years ago we and others showed that most T cells that could react with host peptides are eliminated while the T cells are developing in the thymus, before they can harm their host. It has also been shown that most of the potentially autoreactive T cells that escape elimination are controlled by special mechanisms. Nevertheless, autoimmunity does occur in some individuals. In collaboration with the labs of Drs. Katie Haskins and George Eisenbarth, we are trying to find out how this can happen. Our structural studies suggest that this occurs because the damaging T cells react with versions of the host peptides that are not present in the thymus and therefore could not participate in elimination of the T cells that react with them. In our view, the peptides that drive autoimmunity are, as far as T cells are concerned, foreign peptides.
The ability of a few TCRs to react with peptides from their own host could be used to advantage to deal with cancer. In collaboration with Dr. Jill Slansky’s lab in our Department we are trying to find systematic ways of identifying peptides that would be useful in T cell therapy of tumors, both in experimental mice and in humans.
Finally, MHC proteins are very polymorphic, varying in amino acid sequence from one individual to another. These differences affect the individual’s ability to deal with infections, the individual’s likelihood of developing an autoimmune disease and the individual’s ability to reject transplants. Our new experiments, in association with Dr. Laurent Gapin’s lab in the Department, are aimed at understanding how T cells distinguish systematically between different alleles of MHC.
Building Better Vaccines
Vaccines are crucial to human and animal health. They work by creating large numbers of so-called memory B and T cells that can deal with infection by the agent that was present in the vaccine. For example, the very efficient measles vaccine induces long-lived descendants of B cells, plasma cells, that secrete antibodies against measles virus and prevent infection by the virus itself. To be effective vaccines must contain not only a portion of the target infection but also an adjuvant, material that improves the ability of the vaccine to create B and T cell memory. Many vaccines contain, as an adjuvant, small amounts of so-called alum, insoluble crystals containing aluminum salts. In spite of the fact that alum has been used for many years, safely, as a vaccine adjuvant, little is known about how it performs this task. Other labs and our own have been trying to find out how alum works. The hope is that such knowledge might help us build more effective vaccines using alum, or perhaps other materials, as adjuvants. Solution to the problem has proved difficult but we believe we have some clues from the fact that, when it is injected into the body, alum is rapidly coated with material from the host itself. We have recently used our knowledge of how alum works to design a vaccine that may help to protect humans against recurrent infections with different forms of influenza virus.
B Cells, Gender and Autoimmune Disease
Some autoimmune diseases, for example rheumatoid arthritis (RA), occur much more frequently in females than males. We have recently identified an unusual type of B cell that is found in elderly female, but not male mice and also in autoimmune-prone animals. Because of its incidence in elderly animals we call these B cells Age associated B cells (ABCs). These B cells have also been found by others and ourselves in human patients with RA or scleroderma. Again, they are found in larger numbers in older women with these diseases. ABCs secrete autoantibodies when they are stimulated. Our goals are to find out, in collaboration with the labs of Drs. Pelanda, Torres, Cambier and van Dyk in the Department, how ABCs are induced and how they contribute to disease. Ultimately it may be that ABCs contribute significantly to human autoimmune disease, if this is the case, therapies directed at eradicating these cells are possible, and may help to ameliorate the illnesses.
Other Studies and Technology
Our lab is working on additional subjects, the complexes of proteins related to Bcl-2 that kill cells, vaccines that could be used to sterilize feral cats and dogs and the functions of transcription factors related to helios. We also strive to invent new technologies that help us achieve our experimental goals. Most notable of these is the production of baculovirus-based libraries that allow us to identify peptide-MHC combinations that will engage TCRs or previously unknown specificities. These libraries have greatly helped our studies of the specificity of autoantigen and tumor antigen- specific T cells. As for technologies in general use, our laboratory uses methods ranging from X-ray crystallography and mass spectrometry to production of transgenic and knock out mice to achieve our goals.